
Ohio Bio-Enviro Settlements Inc.
How does a fuel cell work?
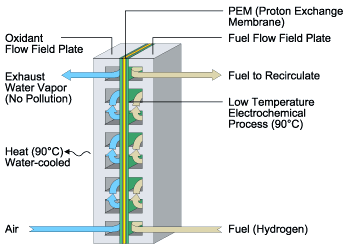
A fuel cell produces electricity by electrochemically combining hydrogen fuel, which can be obtained from fuels such as natural gas, methanol, or petroleum, and oxygen from the air. When fueled by pure hydrogen, heat and pure water vapor are the only by-products from the fuel cell's electrochemical reaction.
What kind of fuel does a fuel cell need to operate?
Fuel cells operate using hydrogen fuel and oxygen from the air. Hydrogen can be obtained through reforming or chemically converting hydrocarbon based fuels such as natural gas, methanol, or gasoline to release the hydrogen so that it can be used by the fuel cell. Fuel cell systems that are not fueled by pure hydrogen will contain a reformer subsystem to convert the source fuel used to the hydrogen required by the fuel cell.
How is hydrogen produced?
Hydrogen can be produced in large amounts from primary energy sources, such as fossil fuels (coal, oil, or natural gas), from a variety of chemical intermediates (refinery products, ammonia, methanol) and from alternative resources such as biomass, biogas, and waste materials. Hydrogen can also be produced by water electrolysis, which uses electricity to split hydrogen and oxygen elements.
 Graciously provided
the following information about PEM fuel cells, if you would like to have more information
about the company and the products they work with please click on their logo to go to
their very informative web page.
Graciously provided
the following information about PEM fuel cells, if you would like to have more information
about the company and the products they work with please click on their logo to go to
their very informative web page.
An Illustration Of How A PEM Fuel Cell Works
![]()
A movie illustrating how Proton Exchange
Membrane (PEM) fuel cells work.
Instructions
To play the movie click on the image above. The audio portion of this movie requires a
sound card on your computer.
The Plug-in
To view the movie you will need the FLASH 3 plugin which can be found at: